Chemistry Form 4 Chapter 7 Acid and Base
A strong acid is one that completely ionises in water to form a large amount of hydrogen ions whereas a weak acid only partially ionises in water and thus produces a small amount of hydrogen ions. As you find a seat in the classroom you read the.
This is the base of Chemistry and what must be noted is that a lot of the important objectives - easy marks questions form the base of this chapter appear every year in Class 11 Chemistry Exam.

. 141 Brønsted-Lowry Acids. An AP site apurinicapyrimidinic site also known as an abasic site. The differences listed above depicts the clear difference between acids and bases which forms part of the chemistry and discussed among students the world over.
HCl H 2 SO 4 HNO 3 are strong acids. The group 15 elements have 5 e-1 s in their valence shell. Acidity is indicated by a pH less than 7 while a pH greater than 7 indicates a base.
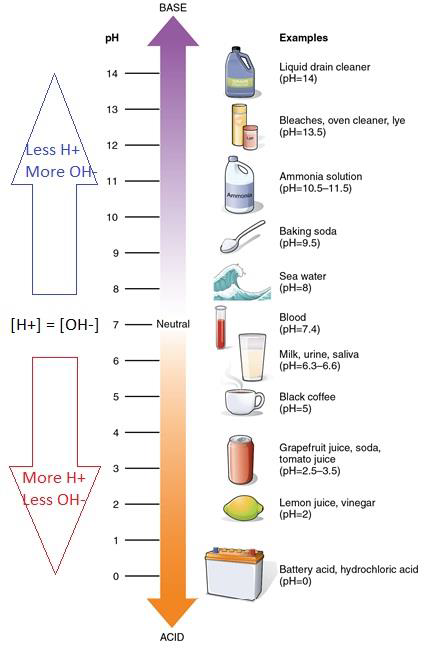
PH is a measurement of the proportion of free hydrogen and hydroxyl ions in water. Class 11th Chemistry Chapter 1 notes cover. Another perhaps simpler way to predict the outcome of this reaction is to use the pK a values of the two acids CH 3 CO 2 H 48 and H 2 O 14 clearly acetic acid is a much stronger acid than water and therefore the equilibrium position for this reaction will lie over to the right in favor of the weakest acid and the weakest base.
What we have done here is change the acetic acid. The other sections that could fit within either a general or organicbiological chemistry chapter are sections 56 redox in organic and biochemistry and 75 energy of biochemical reactions. As we will explore in Chapter 13 methylation of DNA also serves as an important mechanism regulating gene expression.
Why are pentahalides more covalent than trihalidcs. It is difficult to lose 3e-1 s to form E 3 and even more difficult to lose 5e-1 s to form E 5Thus they have very little tendency to form ionic. If you live near a lake a river or an ocean that body of water is not pure H 2 O but most probably a solution.
Recall from Chapter 1 that solutions are defined as homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other. NCERT IN TEXT QUESTIONS 71. Osmium tetroxide OsO 4 is a widely used oxidizing agent for such purpose.
D Change of colour in an acid and a base depends on the type of the indicator. Write reactions of the final alkylation product of aniline with excess of methyl iodide in the presence of sodium carbonate solution. Solutions are all around us.
Important topics covered in NCERT Solutions for class 7. Apurinic and Apyrimidimic AP sites occur due to unstable hydrolysis. You stop to fill your cars gas tank almost making you late for the first day of chemistry class.
If section 46 were moved to chapter 12 then 56 and 75 would likely need to be moved into an organic or biological chemistry chapter as well. Complete the following acid-base reactions and name the products. I CH 3 CH 2 CH 2 NH 2 HCl ii C 2 H 5 3 NHCl Ans.
I All four ii a and d iii b c and d iv only d. Denoted is a quantitative measure of the strength of an acid in solutionIt is the equilibrium constant for a chemical reaction known as dissociation in the context of acidbase reactionsThe chemical species HA is an acid that dissociates into A the conjugate base of. NCERT Solutions CBSE Sample Papers Chemistry Class 12 Chemistry.
Figure 127 Abasic Sites. In chemistry an acid dissociation constant also known as acidity constant or acid-ionization constant. 12-Dihydroxylation the conversion of the CC double bond to 12-diol is an oxidative addition reaction of alkene.
Iv Only d is correct. Potassium permanganate can be used as well though further oxidation is prone to occur to cleave the diol because it is a stronger oxidizing agent 1072. Which of these statements are correct.
Air for example is a solution.

Acids And Bases Basic Introduction Chemistry Youtube
2 4 Acids And Bases Medicine Libretexts

Pin On Study Notes In Form Of Ppt Video

Difference Between Acids And Bases Conduct Science

0 Response to "Chemistry Form 4 Chapter 7 Acid and Base"
Post a Comment